Recently, Xiaohong Wu’s research group from the School of Chemistry and Chemical Engineering of Harbin Institute of Technology and Professor Wang Qing from the National University of Singapore have made important breakthroughs in aqueous redox flow batteries. The obtained results entitled“Lithium Ferrocyanide Catholyte for High-Energy and Low-cost Aqueous Redox Flow Batteries" have been published in Angewandte Chemie International Edition. This work breaks through the solubility limit of ferrocyanide and significantly promotes the commercial application of ferrocyanide/ferricyanide-based redox flow batteries (RFBs). More importantly, the design strategy employed for this active material holds the potential for extension to other aqueous energy storage technologies, with the aim of enhancing energy storage and conversion efficiency.
Redox flow battery, as a prominent technology for large-scale energy storage, plays a crucial role in the storage and utilization of renewable energy sources, such as solar and wind energies. Ferrocyanide/ferricyanide possessing a stable structure, environmentally friendly properties, and affordability has been considered an ideal redox active material in RFBs. However, the limited solubility of ferrocyanide/ferricyanide seriously hampers the energy density of RFBs, emerging as a bottleneck issue that restricts their commercial application.
To address it, according to the previous research results (Joule, 2019, 3, 2255), the team proposed an intramolecular/intermolecular interaction-based molecular design strategy and successfully synthesized lithium ferrocyanide (Li4[Fe(CN)6]) for the first time. It exhibits a significantly improved solubility of 2.32 mol/L at room temperature. The constructed aqueous zinc/iron RFB exhibits high capacity, low cost, and long life. Under neutral and alkaline conditions, the average capacity retention rate of half-cell ARFB is close to 100% and the energy storage capacity of the full RFB reaches 61.64 Ah/L and 56.28 Ah/L respectively. Moreover, the overall chemical cost of this system is as low as $11 per kWh.
Harbin Institute of Technology is the first corresponding affiliation of the paper. Professor Xiaohong Wu from Harbin Institute of Technology and Professor Qing Wang from the National University of Singapore are the co-corresponding authors of the paper. Xiaotong Li, a Ph.D. student from the School of Chemistry and Chemical Engineering, is the first author, while Associate Professor Yuan Yao is the co-first author. Ph.D. students Chenxi Liu, Xin Jia, Jiahuang Jian, and Bao Guo from Wu’s group participated in the research.
The research was funded by the National Natural Science Foundation of China and Heilongjiang Touyan Team.
Paper link: https://onlinelibrary.wiley.com/doi/10.1002/anie.202304667

Solubility and energy storage capacity comparison of various ferrocyanide salts
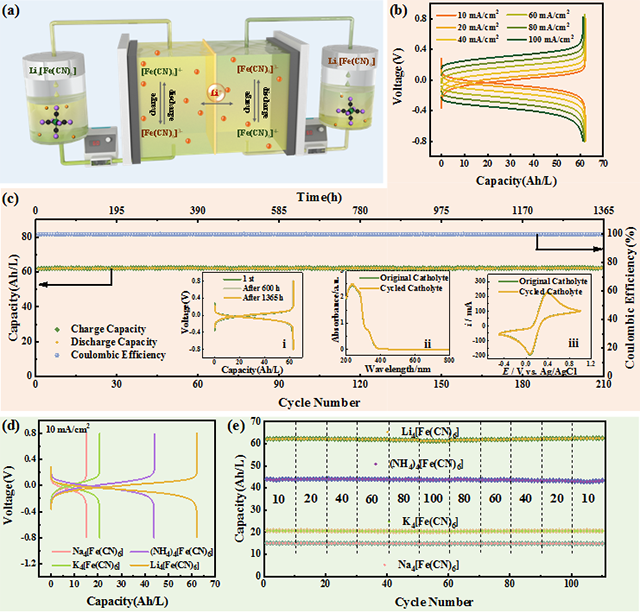
a: Schematic diagram of a neutral half-cell RFB.
b: neutral half-cell RFB charge and discharge curve.
c: 1365 hours of performance testing.
d: Comparison of charge and discharge curves of various ferricyanide salts.
e: Cycling stability of capacity.


